
Playbook
The Recall Prevention Playbook for MedTech Leaders
– How to Achieve Regulatory Enablement and Ship Faster
This is a strategic playbook for MedTech Leaders tired of stagnant processes and ready to achieve regulatory enablement to ship faster.
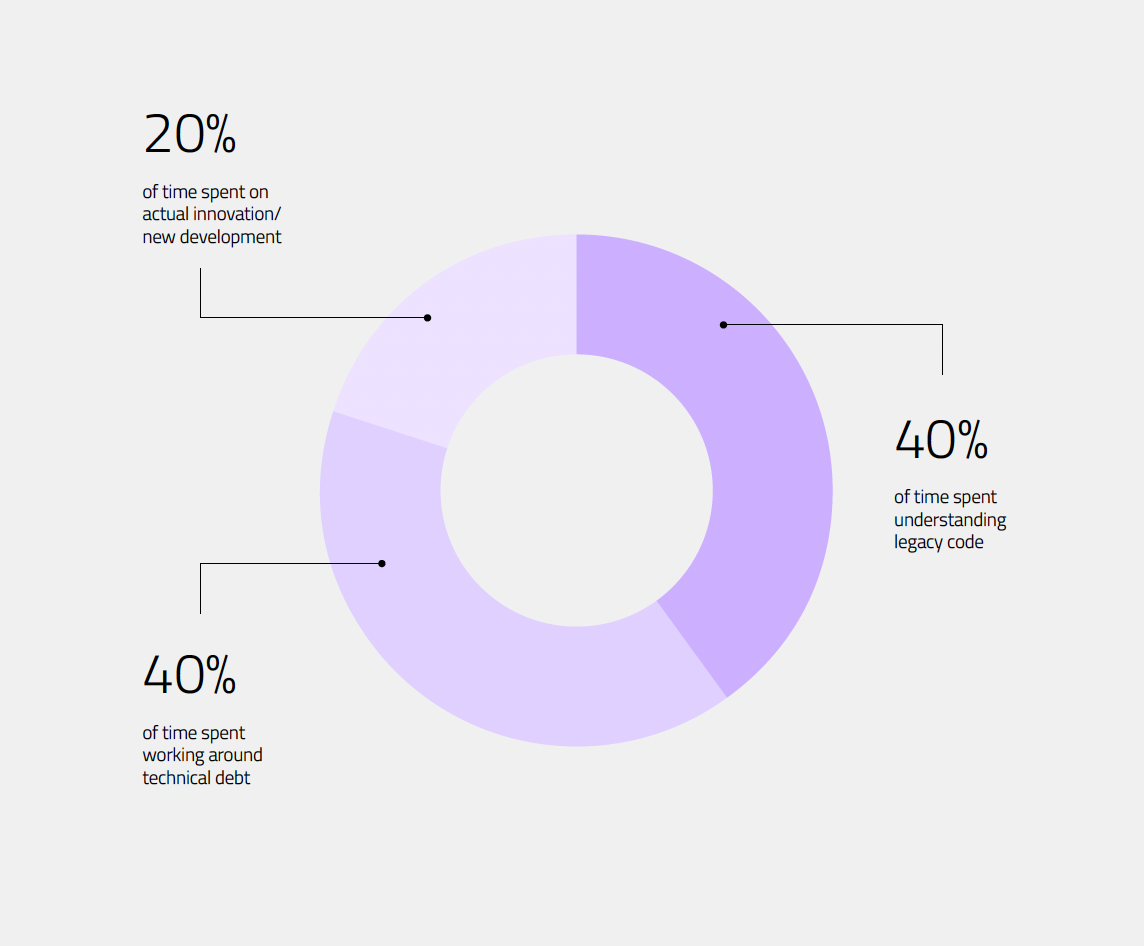
You are delivering groundbreaking medical devices that pass rigorous audits. But the constant scramble for compliance, the deep reliance on "tribal knowledge", and struggling with risks of post-market recalls prove your development process might need rethinking.
This playbook is for leaders who are successfully shipping products but are paying a punishing price for it: you know the current cost of success is unsustainable. This guide provides the framework to shift your focus from building a short-term product to building a long-term, resilient infrastructure for winning products and winning teams.
Who This Strategic Playbook Is For:
This guide is written for the technical experts and engineering leaders who feel the immense weight of complexity, compliance, and constant firefighting.
It's for:
– Technical Leaders who recognize that reacting to audits is a losing strategy and are ready to build compliance into their process.
– Software Architects and Developers who are frustrated by "tribal knowledge" and need the strategic framework to build a resilient, maintainable codebase.
– Decision-Makers ready to transform their software from a 10-year liability into a 10-year strategic asset.
The companies that figure out how to make safe and secure software their superpower will run the next ten years of innovation.
What You Will Master After Reading:
Reframe the Problem:
Understand why the core challenge isn't just passing an audit, but building a resilient infrastructure that makes compliance an automated byproduct of a great process.
Protect The Foundation:
Learn how to master both Architectural Integrity and Code-Level Integrity to protect your product's foundation and the ROI.
Improve Velocity Through Safety and Compliance:
Discover how architectural and code-level integrity work together to enable both development speed and provable functional safety, eliminating the trade-off.
The Path to Regulatory Enablement:
Get the strategy to stop relying on "tribal knowledge" and prove your system is sound, secure, and compliant, accelerating your time-to-market.
A 6-Phase Lifecycle Plan: An actionable plan for embedding quality into every stage of development, from initial concept to post-market surveillance.
From Our Customers
Oncosoft | Built with Qt
Oncosoft uses Qt to boost real-time 3D medical imaging and more than double development efficiency.
Read More
Success Story Fresenius Medical Care | Axivion
Software erosion protection for long-lasting software in the field of medical technology
Read More
Success Story Dentsply Sirona | Axivion
Rule-compliant code and uniform architecture for dentistry technology
Read MoreQt in Medical
Build safe, intuitive, scalable software for medical devices, lab equipment, and healthcare IT. Qt Group offers end-to-end solutions for medical UI design, early usability testing, and faster development with reusable components.
Medical Software Quality Solutions
Qt Group's Software Quality Solutions strengthen Architectural Integrity and Code-Level Integrity, enabling you to detect issues early, streamline documentation, and support secure updates throughout the product lifecycle.


